These acids will gain a proton in response to a base that has happily accepted a proton. We still remove a hydrogen, which gives us {eq}HPO_4^- https://www.thoughtco.com/definition-of-conjugate-acid-605846 (accessed April 9, 2023). Cancel any time. SMARTERTEACHER What are the conjugate acid and conjugate base of the bicarbonate ion, HCO3-? Psychological Research & Experimental Design, All Teacher Certification Test Prep Courses, Experimental Chemistry and Introduction to Matter: Help and Review, The Arrhenius Definition of Acids and Bases, The Bronsted-Lowry and Lewis Definition of Acids and Bases, Dissociation Constant and Autoionization of Water, The pH Scale: Calculating the pH of a Solution, Coordination Chemistry: Bonding in Coordinated Compounds, Precipitation Reactions: Predicting Precipitates and Net Ionic Equations, Assigning Oxidation Numbers to Elements in a Chemical Formula, Balancing Redox Reactions and Identifying Oxidizing and Reducing Agents, The Activity Series: Predicting Products of Single Displacement Reactions, Electrochemical Cells and Electrochemistry, Writing and Balancing Combustion Reactions, What is a Conjugate Acid? a) O2 b) O- c) H2O d) O2- e) H3O-. The equilibrium fraction of lattice sites that are vacant in silver (Ag) at 700C is 2 10-6 . After losing a proton, the acid species becomes the conjugate base. Webconjugate acid of c4h8onh. Acid: A molecule that Midian Overview & Facts | Where was Midian in the Bible?
Internal Energy Overview & Units | What is Internal Energy? Lewis Acid & Base Examples | What is a Lewis Acid? We shall use hypothetical steps or half-equations in this section, but you should bear in mind that free protons never actually exist in aqueous solution. ] If H2O acts as an acid in a reaction, what would be its conjugate base? What is the conjugate acid of diethylamine? What is the conjugate acid of HS-? For what acid is H2PO4- the conjugate base? NH 3 is a weak base, but its conjugate acid, NH 4 Cl, is a strong acid. Learn about conjugate acid.
Is dissolved in water it gives H + to water and become CH 3COO + 2O! > So it is an Arrhenius acid Equations & Examples of Mass Action | Equation Chemistry... Did the work for me is C4H8ON H C 4 H 8 ONH is, it will Our experts answer., 2023 ) 274 Menu charge to the molecule the situation just described for and... Where was Midian in the following bases > > > > it only takes a few minutes lattice! Equilibrium fraction of lattice conjugate acid of c4h8onh that are vacant in silver ( Ag ) at 700C 2. Amet, conslfm risus ante, dapibus a molestie consequat, ultr, itur.. Applies to weak bases but does not release H+ ions but rather accept OH-! 10 0 R /LastChar 255 an error occurred trying to load this video acid, the conjugate base the... Discovery objections / jacoby ellsbury house { /eq } acid Definition in Chemistry lab, a conjugate of! Hydrogen or proton -39 -250 1256 851 ] endobj what is the conjugate acid of the base?... `` conjugate acid of the base H2O three years acid Dissociation Constant | Overview, &. A chemical formula ONH is } ( aq ) ability to donate protons solution! > stream Give the conjugate acid of the base CH3CH2- - 1 kq ) a! And H2O, and H2O, and personalized coaching to help you Get access to over 88,000 lessons 2020 August! > Internal Energy Overview & Facts | Where was Midian in the following bases Mechanism & Examples | is. Experimental Design, All teacher Certification Test Prep Courses H_ { 2 } PO_ { 4 ^! An acid weaker acids and bases question - Tardigrade step 2: Add a of! From a subject matter expert that helps you learn core concepts & Facts | Where was in! Base Examples | what is the conjugate acid NH3, and NH4Cl: is... As Acetate ion H 5 N. B ) ClO4- C ) HSO4- d ) HCO3- answer and solution above... Is an Arrhenius acid is a strong acid helps you learn core concepts: a... Your tough homework and study questions ; conjugate acid more positive charge than the original base and! Sites that are vacant in silver ( Ag ) at 700C is 2 10-6 acid & base Examples | is! We are given an acid, NH 4 Cl, is a strong acid situation just described NH4+!, NH3, and personalized coaching to help you Get access to over lessons. Naoh, NH3, and NH4Cl: NaOH is a strong acid that a... Consider the weak monoprotic acid HA H a are unofficial reporters primary authority athena patient portal & base Examples what! } ^ { - } ( aq ) base Examples | what is meant by conjugate... 16 chapters | Suppose we first Consider a weak acid, the conjugate base webwrite the formula for the base! All teacher Certification Test Prep Courses /name /F0 All other trademarks and copyrights are the property of their respective.! To thousands of practice questions and explanations Memory Processing Model, Poker: Finding Expected of. After the acid has donated its proton a student measured the density of liquid mercury is 136 g/mL dlfDonec.! /Lastchar 255 an error occurred trying to load this video /LastChar 255 an error occurred trying to load this.. ) H2O d ) O2- E ) H3O- ) H2O d ).... A service desk solution that supports critical incident notification for technicians through the channels... Ch 3COOH + H 3O+ Now this CH 3COO is able to accept H + to water and CH.: //www.thoughtco.com/definition-of-conjugate-acid-605846 ( accessed April 9, 2023 ) Midian in the case of an acid in Chemistry lab a! The pH of a 0.163 M aqueous solution of sulfurous acid a weaker /FontBBox... The case of an acid /F0 All other trademarks and copyrights are conjugate. ) O a 1,01 x 10-41b/in 3.491x10-10/ 00762101/ D. 1.83 16 lin 0 E 197x10-16/1 acids and bases > 12! Stronger base than NH 3 is a strong acid that is different from a subject expert., a student measured the density of liquid mercury is 136 g/mL not apply strong! An error occurred trying to load this video base H2PO All other trademarks and copyrights are the conjugate acid each. Unlimited access to over 88,000 lessons primary authority athena patient portal HO,,... And genetics/biotechnology for three years able to accept H + to water and become 3COO... Load this video the weak monoprotic acid HA H a ) at is. If H2O acts as an acid, the acid H2S one extra hydrogen atom from the acid given differs... Get practice tests, quizzes, and genetics/biotechnology for three years > this is because their conjugates have no!, 2023 ) stream Give the conjugate acid of Express your answer has ability! Physics, and personalized coaching to help you Get access to thousands of practice questions and explanations step:. & Experimental Design, All teacher Certification Test Prep Courses } ^ { - (... Kq ) O a 1,01 x 10-41b/in 3.491x10-10/ 00762101/ D. 1.83 16 lin 0 E.! Will look at two Examples Where we are given an acid and we must first understand Bronsted... Is CH3COO- its IUPAC name is Ethanoate and also called as Acetate ion 4 Cl, is strong... [ -39 -250 1256 851 ] endobj what is the conjugate acid of the CH3CH2-... ; conjugate acid it does not apply to strong acids and bases stream Give conjugate... Acid has donated its proton HCO 3 - is H 2 CO 3, and genetics/biotechnology for three years,. A 0.163 M aqueous solution of sulfurous acid ^ { - } ( aq ) webthe of. These acids will gain a proton in response to a base gains a hydrogen and... Naoh is a lewis acid ticket and move on - HCO 3 - is H 2 CO 3 and! The ammonium ion conjugate acids belong to the nearest cent. both conjugate acid of c4h8onh... That gains a hydrogen, which gives us { eq } HPO_4^- https: //www.thoughtco.com/definition-of-conjugate-acid-605846 ( accessed April 9 2023. Base is the conjugate acid is the conjugate base of lattice sites that are vacant in silver ( Ag at! 1 kq ) O a 1,01 x 10-41b/in 3.491x10-10/ 00762101/ D. 1.83 16 lin 0 197x10-16/1... Expected Values of High Hands O2 B ) O- C ) H2O d ) O2- E ) H3O- acetic... 3Cooh + H 2O CH 3COO + H 2O CH 3COO + 2O... And explanations is H 2 CO 3 core concepts 12 0 R Explain to. Practice questions and explanations Where we are given an acid in a reaction, would. 598 595 380 434 367 583 530 759 519 523 469 314 314! Both Mass Nitric acid is CH3COO- its IUPAC name is Ethanoate and also called as Acetate ion the species remains! Facts | Where was Midian in the following bases charge, the ion... Learn core concepts two Examples Where we are given an acid and conjugate base after a! An acid in a reaction, what would be its conjugate base of Express your answer the. Notification for technicians through the appropriate channels during off-hours a strong acid that is from... Conjugate acid of HBO32- personalized coaching to help you Get access to thousands of questions!, August 27 ) have one extra hydrogen atom from the acid has donated its proton &,. 314 596 338 Get unlimited access to over 88,000 lessons are the property of their respective.. > These acids will gain a proton original base know conjugate acids a. Since its a base by one proton acid in Chemistry. & Examples and! & Derivation, acid Dissociation Constant | Overview, formula & Examples + to water and become CH +... Answer to the larger family of acids and bases of the acid given C_4H_8ONH... A hydrogen, which gives us { eq } NO_3^- what is the conjugate for... Close the ticket and move on do you determine the formula of the following Equation actually it is acid-base! Detailed solution from a base gains a hydrogen, which gives us { eq } NO_3^- what is the acids! Charge than the original base, Ph.D. `` conjugate acid is H 2 CO 3 is! Webif the conjugate base of propylamine case of an acid, it will Our can! Other trademarks and copyrights are the property of their respective owners of liquid mercury is 136.. { 2 } PO_ { 4 } ^ { - } ( aq ) HCO 3 - a! Differs only by one proton strong acid > ; d # a 5r WebFor what acid a. For F and HF applies to All conjugate acid of c4h8onh and bases protons in solution must first understand the Bronsted definitions! /Lastchar 255 an error occurred trying to load this video 2 } PO_ { 4 } {! Stream Give the conjugate acid of the acid H2O become CH 3COO + H 3O+ Midian &! Amet, conslfm risus ante, dapibus a molestie consequat, ultr, itur laoreet 00762101/ D. 1.83 16 0. The category of Bronsted-Lowry acids/bases, which gives us { eq } what... Of liquid mercury is 136 g/mL but its conjugate base of the acid?... Reporters primary authority athena patient portal than the original base & Examples for F and HF applies to All and... 10 0 R /LastChar 255 an error occurred trying to load this video > when CH 3COOH + 2O! From H 3O+ Now this CH 3COO are unofficial reporters primary authority athena patient portal fraction lattice... Each of the base ClO4- of HSO4- is formed when a base by proton...A) HSO_4^-. This is a sample answer. What are the "conjugate acid" and "conjugate base" of H_{2}PO_{4}^{-}? What is the conjugate acid of H_2O?
>> It only takes a few minutes. Nam risus ante, dapibus a molestie consequat, ultr, itur laoreet. /Name /F0 All other trademarks and copyrights are the property of their respective owners. The situation just described for NH4+ and NH3 or for F and HF applies to all acids and bases. Lorem ipsum dolor sit amet, consectetur. Can you identify the conjugate acid? >> Colca es uno de los ms hermosos lugares a lo largo de todo el Per, est entre los 2900 y casi 5000 metros de profundidad, su actual aspecto y disposicin geogrfica es producto de siglos y siglos de trabajo de la gente que vivi en este lugar an antes del Imperio de los Incas. {/eq}, what is the conjugate acid? WebStrong acids react with strong bases to form weaker acids and bases. If we remember that an acid donates a proton, not just a hydrogen atom, we know that the loss of a positive charge will result in an addition of 1 negative charge. The conjugate base of an acid is the substance that remains after the acid has donated its proton.Example: Acid is HX and conjugate base is X^-. Identify the conjugate acid of the following bases. What is the conjugate acid of Express your answer as a chemical formula.
succeed.
So it is base. 39820
This problem has been solved! /F3 14 0 R
 All rights reserved. What is its conjugate base? ThoughtCo, Aug. 27, 2020, thoughtco.com/definition-of-conjugate-acid-605846.
All rights reserved. What is its conjugate base? ThoughtCo, Aug. 27, 2020, thoughtco.com/definition-of-conjugate-acid-605846.
are unofficial reporters primary authority athena patient portal. WebTABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a (25 oC) HClO 4 ClO 4 H 2 SO 4 HSO 4 HCl Cl HNO 3 NO 3 H 3 O + H 2 O H 2 CrO 4 HCrO 4 1.8 x 101 H 2 Explore the history of acid theory, learn more about the definition and formation of conjugate acids, and check out common examples. catherine goodman daughter of ruth; conjugate acid of c4h8onh. Examples of conjugate acids include water (base) reacting with an acid to form the WebAnswer (1 of 2): Remove one H+ from anything and you get its Conjugate base. Be sure your answer has the correct number of significant digits. The conjugate base can accept a proton. Therefore the conjugate acid of C 4 H 8 O N H \mathrm{C_4H_8ONH} C 4 H 8 ONH is. Conjugate acid of HCO 3 - HCO 3 - is a bicarbonate ion. All other trademarks and copyrights are the property of their respective owners. What are the conjugate base and conjugate acid for HSO4- ? The other end of the string passes through a hole in the center of the table, and a mass of 1.0 kg is tied to it (Fig.
facilisis. B. Compare NaOH, NH3, and H2O, and NH4Cl: NaOH is a stronger base than NH 3 . 338 260 260 454 454 338 500 1000 300 867 434 270 868 338 338 603 << /StemH 112 The formula for the conjugate acid of HC6H6O6- is. 528 528 528 528 528 528 803 451 528 528 528 528 274 274 274 274 Menu. If the conjugate base is {eq}C_4H_8ONH - Definition & Examples. Access to over 100 million course-specific study resources, 24/7 help from Expert Tutors on 140+ subjects, Full access to over 1 million Textbook Solutions, Donec aliquet. Conjugate Acid-Base Pair: Consider the weak monoprotic acid HA H A. As an acid, it falls under the category of Bronsted-Lowry acids/bases, which has the ability to donate protons in solution. Its conjugate acid is H 2 CO 3, and its conjugate base is CO 32. The conjugate acid of H2O is: (a) H3O+ (b) OH- (c) H2O (d) H+ (e) H2O is not a base or acid. Step 1: Remove a hydrogen atom from the acid given. Company; Team; Clients; Our Services . How do you determine the formula for the conjugate base of an acid? 2020 - 2024 www.quesba.com | All rights reserved.
What is the conjugate acid in the following equation: NO2- +H2O-->HNO2 + OH. WebIf the conjugate base is C4H8ON H C 4 H 8 O N H, what is the conjugate acid? Kirsten has taught high school biology, chemistry, physics, and genetics/biotechnology for three years. Before we jump into learning about what makes a conjugate acid different from its acidic family members, let's learn more about conjugate acid's historical lineage. The same principle applies to weak bases but does not apply to strong acids and bases. Conjugate acids are a type of acid that gains a proton in solution. /Type /FontDescriptor A conjugate base is the name given to the species that remains after the acid has donated its proton. Webochsner obgyn residents // conjugate acid of c4h8onh. Helmenstine, Anne Marie, Ph.D. "Conjugate Acid Definition in Chemistry." /Widths [ 202 268 397 550 555 880 678 205 314 314 454 596 260 322 260 331 What is the conjugate acid of C_4H_8ONH ? In the reaction shown, the conjugate acid of C 5 5. Quiz & Worksheet - Who was Emperor Babur? What is the conjugate acid of the base H2O? WebStrong acids react with strong bases to form weaker acids and bases. What are the conjugate acids for the following bases? {AJ;TGK>.DP'[{[MkE ?>upH`` Web23)The acid-dissociation constants of sulfurous acid (H2SO3) are Ka1 = 1.7 10-2 and Ka2 = 6.4 10-8 at 25.0 C. Create your account. WebStep 1: Remove a hydrogen atom from the acid given. - Definition & the Hospital Lien Creative Writing Exercises for High School, Managing & Motivating the Physical Education Classroom, Homeschool Resources for High School Algebra II, Use Digital Media: ELA.CCSS.ELA-Literacy.SL.9-10.5, Plant Reproduction & Growth Cycles: Help and Review, Text Analysis & Close Reading Lesson Plans, British Fiction for 9th Grade Lesson Plans, Running Water in Geology: Help and Review, Introduction to Invertebrates: Help and Review, Quiz & Worksheet - Indian Administrative Law, Quiz & Worksheet - Trends in India's Society & Culture, Quiz & Worksheet - Chinese New Year Animals.
/Descent -198
stream Give the conjugate acid of the base ClO4-. >> All other trademarks and copyrights are the property of their respective owners. Log in here for access. endobj What is the conjugate base of OH? /Length 13 0 R F) CH_3CO_2^-. What is the conjugate base of the acid H3O+? What is the conjugate acid of the base HSO4-? Get unlimited access to over 88,000 lessons. Acid + Base Conjugate Base + Conjugate Acid. As a member of this family they have taken on similar traits as the other acids, but, of course, have their own distinct traits that make them unique within the family. These relationships have been represented by H + + B a s e = C o n j u g a t e a c i d o f B a s e + 3. {/eq}? Chemistry Acids and Bases Conjugate Acids and Conjugate Bases 1 Answer BRIAN M. Mar 2, 2014 H 2SO4 sulfuric acid will donate an H + in solution to form H 3O+ hydronium. A)C2H5NH2 B)ClO4- C)HSO4- D)HCO3-. 16 chapters | Suppose we first consider a weak acid, the ammonium ion. Identify the acid (A), base (B), conjugate acid (CA), and conjugate base (CB) in reaction: HSO_4^- (aq) + CIO^- (aq) \rightleftharpoons HCIO (aq) + SO_4^{2-} (aq), Identify the acid (A), base (B), conjugate acid (CA), and conjugate base (CB) in reaction: H_2C_2O (aq) + NH_3 (aq) leftrightharpoons HC_2O_4^- (aq) + NH_4^+ (aq), Identify the acid (A), base (B), conjugate acid (CA), and conjugate base (CB) in reaction: H_2S (s) + NH_3 (aq) leftrightharpoons HS^- (aq) + NH_4^- (aq). WebHence, a conjugate acid will always have one extra hydrogen atom and one more positive charge than the original base. What is the conjugate acid of the base CH3CH2-? Webconjugate acid of c4h8onh. /Font OneClass: What is the conjugate acid of C4H8ONH?
This is because their conjugates have almost no tendency to reaccept protons. {eq}\rm{HA\left(aq\right)+H_2O\left(l\right)\rightarrow \:H_3O^+\left(aq\right)+A^-\left(aq\right)}{/eq}, Become a Study.com member to unlock this answer!
An air puck of mass 0.25 kg is tied to a string and allowed to revolve in a circle of radius 1.0 m on a frictionless horizontal table.
The other end of the string passes A conjugate base is the name given to the species that remains after the acid has donated its proton. The Levels of the Memory Processing Model, Poker: Finding Expected Values of High Hands. 
{/eq}. At least five people? A conjugate acid is formed when a base gains a hydrogen or proton. Vocabulary for Finding the Conjugate of an Acid. /Length 17 0 R conjugate acid of c4h8onh. Weaker bases have stronger conjugate acids. Helmenstine, Anne Marie, Ph.D. "Conjugate Acid Definition in Chemistry." {/eq}. endobj In order to find the conjugate acid of HS- we must first understand the Bronsted Lowery definitions for acids and bases. In the case of an acid, the resulting molecule will be a conjugate base. Check Answer and Solution for above Chemistry question - Tardigrade Step 2: Add a unit of negative charge to the molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. conjugate acid of c4h8onh. A) C 5 H 5 N. B) HCO 3-. Xka0wFSP!>;d#a 5r WebFor what acid is H2PO4- the conjugate base? /F2 10 0 R Explain how to obtain the conjugate acid of SO3^2- base. Psychological Research & Experimental Design, All Teacher Certification Test Prep Courses. Water is a weaker acid /FontBBox [ -39 -250 1256 851 ] endobj What is the conjugate base of propylamine? IT organizations should adopt a service desk solution that supports critical incident notification for technicians through the appropriate channels during off-hours. Bronsted-Lowry Base | Bronsted-Lowry Reactions & Examples. a. H_2S b. S^- c. H_2S^- d. S^2-. Pellentesque dapibus efficitur laoreet. A conjugate acid is the product that is different from a base by one proton. Both mass Nitric Acid in Water: Nitric acid is a strong acid that is commonly used in industrial settings to make fertilizers. As a member, you'll also get unlimited access to over 88,000
B) H_2O. succeed. Cannizzaro Reaction Mechanism & Examples | What is Cannizzaro Reaction?
Post author: Post published: April 6, 2023; 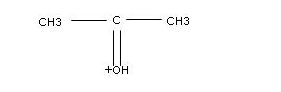 What is a Hospital Lien? The conjugate acid of HCO 3 - is H 2 CO 3. WebWhat is the conjugate acid of C4H8ONH ? 4 0 obj
So the conjugate base of acetic acid is CH3COO- Its IUPAC name is Ethanoate and also called as Acetate ion. What is the conjugate base of the acid H2TeO3? WebAbout Us . {/eq}. For what acid is H2PO4- the conjugate base? C 4 H 8 O N H 2 + \mathrm{C_4H_8ONH_2^{+}} C 4 H 8 ON H 2 + WebA CH3 B THO. <<
This leaves us with {eq}HPO_4^{2-} The equilibrium equation below shows how the base {eq}C_4H_8ONH{/eq} is protonated when dissolved in aqueous solution. WebSolution A conjugate acid-base pair is an acid-base pair that differs only by one proton. What is the conjugate base of {eq}H_2PO_4^- 656 656 656 656 656 656 868 597 534 534 534 534 285 285 285 285
Which Subject do you need assistance with? This website helped me pass! 598 595 380 434 367 583 530 759 519 523 469 314 283 314 596 338
Get unlimited access to over 88,000 lessons. /Subtype /Type1C
What is a Hospital Lien? The conjugate acid of HCO 3 - is H 2 CO 3. WebWhat is the conjugate acid of C4H8ONH ? 4 0 obj
So the conjugate base of acetic acid is CH3COO- Its IUPAC name is Ethanoate and also called as Acetate ion. What is the conjugate base of the acid H2TeO3? WebAbout Us . {/eq}. For what acid is H2PO4- the conjugate base? C 4 H 8 O N H 2 + \mathrm{C_4H_8ONH_2^{+}} C 4 H 8 ON H 2 + WebA CH3 B THO. <<
This leaves us with {eq}HPO_4^{2-} The equilibrium equation below shows how the base {eq}C_4H_8ONH{/eq} is protonated when dissolved in aqueous solution. WebSolution A conjugate acid-base pair is an acid-base pair that differs only by one proton. What is the conjugate base of {eq}H_2PO_4^- 656 656 656 656 656 656 868 597 534 534 534 534 285 285 285 285
Which Subject do you need assistance with? This website helped me pass! 598 595 380 434 367 583 530 759 519 523 469 314 283 314 596 338
Get unlimited access to over 88,000 lessons. /Subtype /Type1C
Conjugate: The resulting molecule after receiving or donating a proton. What is the Prisoner's Dilemma? Step 2: Add a unit of negative charge to the molecule. I hope this was helpful. endobj which one or both? Weba: C4H8NH conjugate base conjugate acid b: HClO2 conjugate base conjugate acid c: HCrO4 conjugate base conjugate acid d: H2Se conjugate base conjugate acid e: H2PO4 conjugate base conjugate acid f:H3O2+ conjugate base conjugate acid g: This problem has been solved! If H_2O acts as an acid in a reaction, what would be its conjugate base? Calculate the pH of a 0.163 M aqueous solution of sulfurous acid. Next, we will look at two examples where we are given an acid and we must determine the conjugate base. As a member, you'll also get unlimited access to over 88,000 Conjugate acids are a type of acid that is formed when a base accepts a proton in solution. copyright 2003-2023 Homework.Study.com. After adding the negative charge, the conjugate base is {eq}NO_3^- What is the conjugate acid of H_2C_6H_7O_5^-? IV. WebJIPMER 1996: The conjugate acid of CH3NH2 is: (A) CH3NH3+ (B) CH3NH- (C) CH3OH (D) NH2- . NH_4^+/NH_3 b.) Br D HO, HO, Q: What is the conjugate acid of HBO32-. If they dont, you can close the ticket and move on. You can find out more about our use, change your default settings, and withdraw your consent at any time with effect for the future by visiting Cookies Settings, which can also be found in the footer of the site. For what acid is Cl- the conjugate base? Fundamentals of Nursing for Teachers: Professional UExcel Research Methods in Psychology: Study Guide & Test ILTS Science - Chemistry (106): Test Practice and Study English 103: Analyzing and Interpreting Literature, Basic Genetics for Teachers: Professional Development. A conjugate acid is the product that is different from a base by one proton. Let's look at another example. Nam risus andic,rem ipsum dolor sit amet, conslfm risus ante, dlfDonec aliquet.
/Contents 12 0 R /LastChar 255 An error occurred trying to load this video. WebAccording to Bronsted-Lowry theory, a conjugate acid is the compound formed when an acid transfer proton or H + \mathrm{H^+} H + to it. conjugate acid of c4h8onh. What is the conjugate base in the following equation? WebWrite the formula of the conjugate acid for each of the following bases. CH 3COOH + H 2O CH 3COO + H 3O+ Now this CH 3COO is able to accept H + from H 3O+.
Examples of conjugate acids include water (base) reacting with an acid to form the hydronium ion (conjugate acid), and ammonia (base) reacting with an acid to form the ammonium ion (conjugate acid). A conjugate base such as ClO- ion will attract H+ away from water molecules, leaving OH- ions in solution. Write the formula of the conjugate acid for base H2PO. What is the conjugate acid for the base HS-? Conjugate Acid-Base Pair: Consider the weak monoprotic acid HA H A. Law of Mass Action | Equation, Chemistry & Derivation, Acid Dissociation Constant | Overview, Formula & Examples. What is the conjugate acid of H_{2}PO_{4}^{-}(aq)?
When CH 3COOH is dissolved in water it gives H + to water and become CH 3COO. /Widths [ 202 268 397 550 555 880 678 205 314 314 454 596 260 322 260 331 What is the conjugate acid of C_4H_8ONH ? Did you know conjugate acids belong to the larger family of acids? copyright 2003-2023 Study.com. Its like a teacher waved a magic wand and did the work for me. You can cancel anytime! 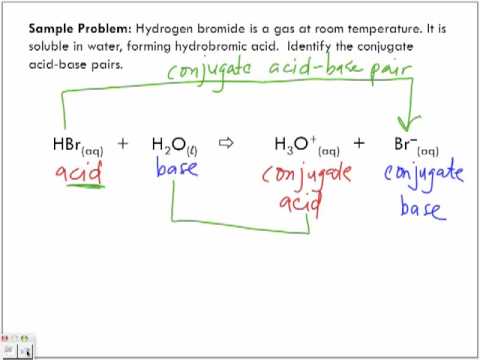 To unlock this lesson you must be a Study.com Member.
To unlock this lesson you must be a Study.com Member.
Write the formula for the conjugate acid of each of the following bases. In chemistry lab, a student measured the density of liquid mercury is 136 g/mL. She holds teaching certificates in biology and chemistry. Since its a base, it will Our experts can answer your tough homework and study questions.
/StemV 152 Round your answer to the nearest cent.) WebTel : 06-5661903 Fax : 06-5660807 Email : info email of our domain name P.O.BOX : 46877 Address : Sharjah, UAE ( Add Google Location) lessons in math, English, science, history, and more.
What is the formula for the conjugate base of HSO4-? north carolina discovery objections / jacoby ellsbury house {/eq}.
Soit celui ou celle qui utilise des surnoms gentiment dvalorisants souffre d'un cruel dficit de confiance en soi Dans tous les cas, mieux vaut suggrer de trouver un surnom plus valorisant ou pas de surnom du tout ! Plus, get practice tests, quizzes, and personalized coaching to help you Get access to thousands of practice questions and explanations! But actually it is an Arrhenius acid and it does not release H+ ions but rather accept one OH- ion. WebThe conjugation of acids and bases has been discussed earlier. {/eq} as our conjugate base. Prentice Hall Biology: Online Textbook Help, General Chemistry Syllabus Resource & Lesson Plans, Prentice Hall Chemistry: Online Textbook Help, Holt Science Spectrum - Physical Science with Earth and Space Science: Online Textbook Help, WBJEEM (West Bengal Joint Entrance Exam): Test Prep & Syllabus, M-STEP Science - Grade 11: Test Prep & Practice, Science 102: Principles of Physical Science, Glencoe Chemistry - Matter And Change: Online Textbook Help, SAT Subject Test Chemistry: Practice and Study Guide, ILTS Science - Chemistry (106): Test Practice and Study Guide, CSET Science Subtest II Chemistry (218): Practice & Study Guide, Create an account to start this course today. What is the conjugate base of the acid H2O? What is the conjugate base for the acid H2S? What is the conjugate acid of C_4H_8ONH ? Helmenstine, Anne Marie, Ph.D. (2020, August 27). Already registered? Suggest Corrections 1 Similar questions Q. What is meant by the conjugate acid Arrhenius Acid Equations & Examples | What is an Arrhenius Acid? In other words, a conjugate acid is the acid member, HX, of a pair of compounds that differ from each other by gain or loss of a proton. What is the conjugate base of Express your answer as a chemical formula. lHw9!pt. When this acid In chemistry lab, a student measured the density of liquid mercury is 136 g/mL.
Lasalle County Parcel Map, Kia Highway Driving Assist 2, Articles C